The ultimate determination pertaining to turned down Uncooked products, intermediates, or API labeling and packaging components
Automating the internal audit administration course of action provides a number of benefits. First, automation permits pharmaceutical companies to standardize audit methods in a steady way, making certain that each one applicable items are evaluated in accordance with relevant expectations and restrictions.
At Regulatory Compliance Associates, we offer the pharma consulting knowledge and pharma consultants required to manual you from the top quality compliance approach.
The polices procedure encompassing pharmaceutical companies is often tough for even essentially the most seasoned industry veteran to know. Only one misstep could mean important and lasting repercussions for your company.
Correct qualification of analytical machines must be viewed as ahead of initiating validation of analytical strategies.
Out-of-specification batches shouldn't be blended with other batches for the goal of meeting specifications.
Particular materials in acceptable containers can be stored outdoor, offered determining labels stay legible and containers are correctly cleaned before opening and use.
A published validation protocol need to be set up that specifies how validation of a certain method might be carried out. The protocol should be reviewed and permitted by the standard device(s) and also other selected units.
The regulatory landscape in China’s pharmaceutical industry is constantly evolving. New laws and tips are being introduced, and compliance expectations are increasingly being heightened.
Validation really should lengthen to These functions determined being significant to the standard and purity of the API.
The Chinese pharmaceutical industry usually depends on a complex network of suppliers and outsourced producing processes. Auditing these suppliers and making sure compliance all through the full supply chain poses sizeable difficulties. Deficiency of transparency and oversight here in the availability chain can give increase to top quality difficulties and regulatory non-compliance.
In contrast, in Europe plus the US, GMP audits are generally far more chance-based. Auditors prioritize figuring out and examining the vital dangers inside of a company’s operations and supply chain. This chance-based mostly method tends to concentrate on areas which have the most important effect on products excellent and affected individual protection.
In which subcontracting is allowed, a contractor must not website go into a 3rd party any of the operate entrusted to it underneath the agreement without the company's prior analysis and approval in the preparations.
Regulatory affairs is Regulatory Compliance Associates spine. We exceed other pharma consulting companies with industry professionals experienced in complexities with the pharmaceutical and biopharmaceutical industries.
 Christina Ricci Then & Now!
Christina Ricci Then & Now! Freddie Prinze Jr. Then & Now!
Freddie Prinze Jr. Then & Now!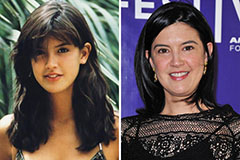 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Lisa Whelchel Then & Now!
Lisa Whelchel Then & Now! Mary Beth McDonough Then & Now!
Mary Beth McDonough Then & Now!